COVID-19 Serology (Anti-SARS-CoV-2 IgG Assay)
The Anti-SARS-CoV-2 IgG assay determines the presence of IgG antibody to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which causes COVID-19 syndrome. It can be used as an aid in the diagnosis of SARS-CoV-2 infection in conjunction with the clinical presentation and other laboratory tests. Results from the Anti-SARS-CoV-2 IgG assay should not be used as the sole basis for diagnosis.
Anti-SARS-CoV-2 IgG assay will only be performed on clinical cases approved by Medical Officer of Health (MOH) on Call. It is not intended to be used to detect the presence of antibody post-vaccination. Clinical scenarios of severe illness with negative or inconclusive COVID19 PCR tests, where serology results may be helpful for clinical management and/or public heath action, will be considered following consultation and approval by the MOH, prior to specimen collection. Specimens submitted for testing without prior approval will be rejected. Please contact MOH at 1-866-270-7437 for test approval.
Aliases
- COVID-19 serology
- Anti-SARS-CoV-2 IgG
Reporting Name
Anti-SARS-CoV-2 IgG assay
- Human serum collected in serum-separating tube (SST).
- To ensure accuracy of results, serum specimens should be free of fibrin, red blood cells or other particulate matter.
Minimum Specimen Volume
Minimum of 1 ml of serum is required
Specimen Collection, Transportation and Storage
Serum can be stored at 2-8°C and shipped on ice pack if delivery is within 24h post-collection. Otherwise, freeze (-20°C or colder) and ship frozen on dry ice.
Rejection Criteria
- Serum not meeting the requirements of minimum volume, transportation and storage
- Specimens that have been pooled (different collection times)
- Specimens that have been heat-inactivated
- Bodily fluids other than serum or plasma
- No MOH approval
- Hemolyzed, icteric, lipemic, or microbially contaminated serum is not recommended for testing and will not be processed. Assay has not been validated for cadaveric samples.
Specimen Retention
Specimens where COVID-19 serology has been performed are stored for 5 years if positive and 1 year if negative.
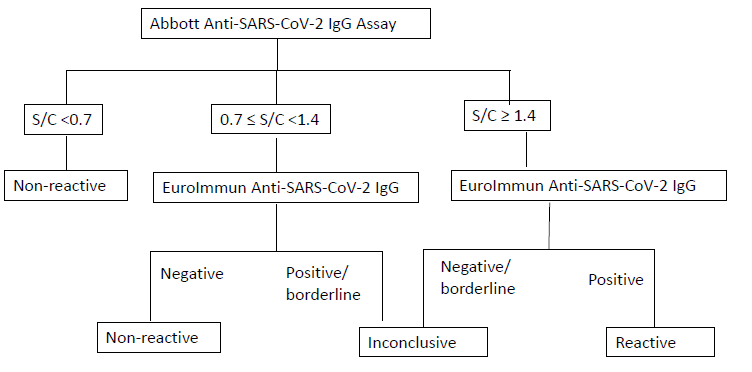
COVID-19 serology test is a two-tiered algorithmic approach, i.e., testing with Abbott SARS-CoV-2 assay (CMIA method, detecting antibody against the nucleocapsid proteins of SARS-CoV-2 virus) followed by EuroImmun SARS-CoV-2 IgG Assay (EIA method, detecting antibody against the S1 domain of the spike protein of SARS-CoV-2 virus).
| SARS-CoV-2 IgG Result | Interpretation |
| Nonreactive | · SARS-CoV-2 IgG should NOT be used to infer immunity status or infectivity.
· The absence of IgG does not rule out current or previous exposure and may occur if the specimen is collected too soon following infection, in immunocompromised patients, or if the patient is younger than 12 months old. · If there is a high clinical suspicion of a SARS-CoV-2 infection and the blood was collected within 2 weeks of the exposure, consider recollecting and testing in 2-3 weeks. · If the patient is currently symptomatic, consider testing a respiratory specimen using a molecular COVID-19 assay. |
| Inconclusive | · SARS-CoV-2 IgG should NOT be used to infer immunity status or infectivity.
· If there is a high clinical suspicion of a SARS-CoV-2 infection and the blood was collected within 2 weeks of the exposure, consider recollecting and testing in 2-3 weeks. · If the patient is currently symptomatic, consider testing a respiratory specimen using a molecular COVID-19 assay. |
| Reactive | · SARS-CoV-2 IgG should NOT be used to infer immunity status or infectivity.
· The presence of IgG may indicate previous or recent exposure to SARS-CoV-2 or vaccination. Results should be interpreted in the context of clinical and exposure history. · The IgG antibody detected may represent maternal transfer of antibodies which may persist up to 12 months. · False-positives may occur from cross-reaction due to presence of other antibodies or medical conditions. · If the patient is currently symptomatic, consider testing a respiratory specimen using a molecular COVID-19 assay. |
Both assays were validated at PHML with 160 patient specimens including 49 of which were SARS-CoV-2 positive RT-qPCR confirmed cases and 111 were historic serological specimens collected prior to the emergence of SARS-CoV-2.
| Two Tier IgG | Confirmed Positive | 47 | 0 | 47 |
| Confirmed Negative | 0 | 109 | 109 | |
| Inconclusive | 2 | 2 | 4 | |
| Total | 49 | 111 | 160 | |
| Sensitivity (Calculated with inconclusives counted as positive results) | 100.0 (92.8-100.0) | Specificity | 98.2 (93.6-99.8) | |
| Sensitivity (Counted with inconclusives counted as negative results) | 95.9 (86.0-99.5) | Specificity | 100.0 (96.7-100.0) | |
Limitation: No false positives were found by the two tier testing on the same sample of this validation, but may occur rarely.
| Status | Days* | Turn-Around-Time (TAT)* |
| Require MOH approval | Monday – Friday | 72h if approved |
*Please note that test schedules and TAT may change due to equipment maintenance or statutory holidays.
Method Description
Chemiluminescent microparticle immunoassay (CMIA) – Abbott SARS-CoV-2 IgG Assay
Enzyme immunoassays (EIA) – EuroImmun SARS-CoV-2 IgG Assay
Performing Laboratory Location
This test is performed at the Newfoundland & Labrador Public Health Microbiology Laboratory in St. John’s.