HBsAg (Hepatitis B surface antigen with autoreflex Neutralization)
Useful For
Detection of active viral replication in acute and chronic Hepatitis B virus infection.
Testing Algorithm
Reporting Name |
Available separately |
Always performed |
| HBsAg | NO | YES |
| Auto-reflex: HBsAg Neutralization | NO | NO |
Indications for Testing
HBsAg is included in the following investigations/profiles (see Special Instructions):
· Acute Hepatitis Screen (unknown etiology)
· Acute Hepatitis B Follow-up
· Chronic Hepatitis Screen (unknown etiology)
· Previous Hepatitis Exposure
Clinical Information
Several HBV-specific proteins can be detected in patient serum after infection. A marker for active viral replication is the detection of HBsAg during primary infection. HBsAg is made in large excess by infected host cells and are released into the serum during active infection. The presence of HBsAg in the serum of a patient indicates high infectivity. Patients who resolve an acute infection eventually produce anti-HBs. However, when HBsAg is present, anti-HBs may be masked due to binding with HBsAg.
Specimens initially reactive for HBsAg are repeated in duplicate. If reactive, specimens are subjected to confirmation of HBsAg by neutralization employing anti-HBsAg. Only confirmed results are reported as reactive.
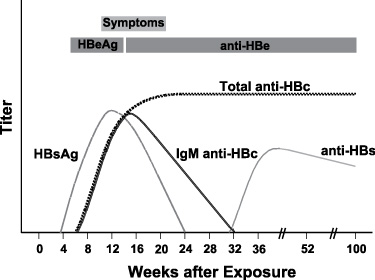
Typical sequence of serologic markers in patients with acute HBV infection with resolution of symptoms (Horvat, R. T., and Tegtmeier, G. E. 2007).
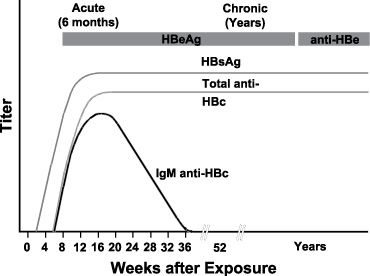
Typical sequence of serologic markers in patients with HBV infection that progresses to chronicity. In patients with chronic HBV infection, both HBsAg and IgG anti-HBc remain persistently detectable, generally for life. HBeAg is variably present in these patients (Horvat, R. T., and Tegtmeier, G. E. 2007).
Hepatitis B virus (HBV) causes a wide spectrum of manifestations ranging from asymptomatic seroconversion, sub-acute illness with non-specific symptoms (e.g. anorexia, nausea, or malaise) or extrahepatic symptoms, and clinical hepatitis with jaundice, to fuliminant fatal hepatitis. Only 10% of children and 50% of adults will exhibit symptoms. An acute illness may last up to three months with a fatality of 1-2%. In most acute cases HBsAg serum levels are positive initially, resolve and the individual develops anti-HBs which confers immunity.
HBV occurs worldwide, and is endemic in some Asian countries. In Canada the incidence of acute hepatitis B is estimated to be 2.3 per 100,000. In developed countries exposure to HBV may be common in certain high-risk groups such as injection drug users, person who have multiple sex partners, men who have sex with men, sex with HBV-infected persons and having a hepatitis B carrier in the family. The prevalence of chronic hepatitis B varies in different populations.
Chronic HBV infection is found in 0.5 % of adults in North America. After acute HBV infection, the risk of developing chronic infection varies with age; infants infected at birth have a 90% chance of becoming a chronic carrier. A chronic carrier is one who retains HBsAg positivity six months after the initial infection. These individuals are always infectious. Hepatocellular carcinoma and hepatic cirrhosis is likely to result in the premature death of 15-25% of those who have chronic HBV.
Reference Values
HBsAg: Non-reactive
Interpretation
For diagnostic purposes, and to stage the disease, results should be used in conjunction with patient history and other hepatitis markers for diagnosis of acute or chronic infection (see special instructions).
A REACTIVE result indicates the presence of actively replicating virus. Patients may be acutely or chronically infected, and are infective.
A NON-REACTIVE result indicates absence of actively replicating virus. It does not rule out chronic infection.
See Special Instructions for PHL recommended diagnostic approach to hepatitis.
Clinical Reference
Curry, M. P., and Chopra, S. 2010. Acute Viral Hepatitis, p. 1577-1592. In Mandell, D., Bennett, J. E., and Dolin, R. Principles and practice of infectious diseases, 7th ed., vol. 2. Churchill Livingstone, Elsevier, Philadelphia, PA.
Horvat, R. T., and Tegtmeier, G. E. 2007. Hepatitis B and D Viruses, p. 1641-1659. In Murray, P. R., Baron, E. J., Jorgensen, J. H., Landry, M. L., and Pfaller, M. A. Manual of Clinical Microbiology, 9th ed., vol. 2. ASM Press, American Society for Microbiology, Washington, DC.
James Koziel, M., and Thio, C. L. 2010. Hepatitis B Virus and Hepatitis Delta Virus, p. 2059-2086. In Mandell, D., Bennett, J. E., and Dolin, R. Principles and practice of infectious diseases, 7th ed., vol. 2. Churchill Livingstone, Elsevier, Philadelphia, PA.
Abbott. 2008. Architect System HBsAg: package insert. Abbott Laboratories, Wiesbaden.