Anti-HBs (Hepatitis B immune status/Anti-Hepatitis B Surface antibody)
Reporting Name
Useful For
Detection of antibody to Hepatitis B surface antigen as a measure of vaccine-induced immunity and immunity due to infection.
Testing Algorithm
| Reporting Name | Available separately | Always performed |
| Anti-HBs | YES | YES |
Indications for Testing
· Anti-HBs should be ordered to determine immune status to HBV and is included as part of the following investigations/orders (see Special Instructions)
· Acute Hepatitis B Follow-up
· Chronic Hepatitis Screen (unknown etiology)
· Previous Hepatitis Exposure
Method Name
Chemiluminescent Microparticle Immunoassay (CMIA)
Reporting Name
Anti-HBs mIU/ml (milli-international units per milliliter)
Aliases
Anti-HBs Surface antibody
HBV Hepatitis B virus
Specimen Required
Serology: Suitable specimens are individual samples (human sera or EDTA/heparinized/citrated plasma) obtained by standard laboratory techniques.
Blood
Container/Tube: Serum separator (SST) or Plain Red-top tube(s)
Specimen Volume: 5 mL of whole blood
Specimen Minimum Volume: 0.3 mL
Transport Temperature
| Specimen | Room temperature | Refrigerated | Frozen |
| Serum | NO | YES* | YES** |
*The samples should be stored for not more than 2 days at 2-8°C.
**For longer delay, freeze at -20°C or below and transport on dry ice.
Reject Due To
| Specimens other than | Serum |
| Anticoagulants | REJECT |
| Hemolysis | REJECT |
| Lipemia | REJECT |
| Icteric | REJECT |
Useful For
Detection of antibody to Hepatitis B surface antigen as a measure of vaccine-induced immunity and immunity due to infection.
Clinical Information
Anti-HBs is a key serological marker for both vaccine-induced immunity and immunity due to infection. Anti-HBs is also used to monitor the convalescence and recovery of HBV infected individuials. The presence of anti-HBs after acute HBV infection and loss of Hepatitis B virus surface antigen (HBsAg) can be a useful indicator of disease resolution. Detection of of anti-HBs in an asymptomatic individual may indicate previous exposure to HBV.
Postvaccination testing for the presence of anti-HBs is not recommended for infants, children, and most adults. Exceptions to this rule are infants born to mothers who are HBsAg-positive, immunocompromised patients, dialysis patients, and healthcare workers whose successful vaccination needs to be confirmed. Infants born to HBV-positive mothers should be testing for anti-HBs after reaching 2 years of age due to passive antibody from the mother.
HBV “escape” mutants in which the HBsAg is altered can result in HBV infections without detectable anti-HBs.
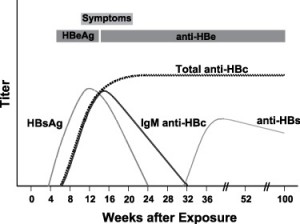
Typical sequence of serologic markers in patients with acute HBV infection with resolution of symptoms (Horvat, R. T., and Tegtmeier, G. E. 2007).
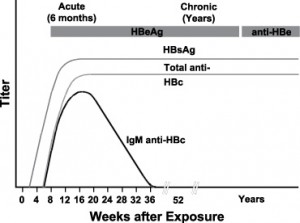
Typical sequence of serologic markers in patients with HBV infection that progresses to chronicity. In patients with chronic HBV infection, both HBsAg and IgG anti-HBc remain persistently detectable, generally for life. HBeAg is variably present in these patients (Horvat, R. T., and Tegtmeier, G. E. 2007).
Hepatitis B virus (HBV) causes a wide spectrum of manifestations ranging from asymptomatic seroconversion, sub-acute illness with non-specific symptoms (e.g. anorexia, nausea, or malaise) or extrahepatic symptoms, and clinical hepatitis with jaundice, to fuliminant fatal hepatitis. Only 10% of children and 50% of adults will exhibit symptoms. An acute illness may last up to three months with a fatality of 1-2%. In most acute cases HBsAg serum levels are positive initially, resolve and the individual develops anti-HBs which confers immunity.
HBV occurs worldwide, and is endemic in some Asian countries. In Canada the incidence of acute hepatitis B is estimated to be 2.3 per 100,000. In developed countries exposure to HBV may be common in certain high-risk groups such as injection drug users, person who have multiple sex partners, men who have sex with men, sex with HBV-infected persons and having a hepatitis B carrier in the family. The prevalence of chronic hepatitis B varies in different populations.
Chronic HBV infection is found in 0.5 % of adults in North America. After acute HBV infection, the risk of developing chronic infection varies with age; infants infected at birth have a 90% chance of becoming a chronic carrier. A chronic carrier is one who retains HBsAg positivity six months after the initial infection. These individuals are always infectious. Hepatocellular carcinoma and hepatic cirrhosis is likely to result in the premature death of 15-25% of those who have chronic HBV.
Reference Values
Vaccinated individuals
Anti-HBs: ≥ 10 mIU/ml
Non-vaccinated individuals
Anti-HBs: 0 mIU/ml
Interpretation
For diagnostic purposes, and to stage the disease, results should be used in conjunction with patient history and other hepatitis markers for diagnosis of acute or chronic infection (see special instructions, Hepatitis Interpretive).
For determination of immunity the World Health Organization (WHO) recognizes a level of 10 mIU/ml of anti-HBs as protective. See Special Instructions for PHL recommended diagnostic approach to hepatitis.
Clinical Reference
Abbott. 2008. Architect System Anti-HBs: package insert. Abbott Ireland, Diagnostics Division. Ireland.
Curry, M. P., and Chopra, S. 2010. Acute Viral Hepatitis, p. 1577-1592. In Mandell, D., Bennett, J. E., and Dolin, R. Principles and practice of infectious diseases, 7th ed., vol. 2. Churchill Livingstone, Elsevier, Philadelphia, PA.
Horvat, R. T., and Tegtmeier, G. E. 2007. Hepatitis B and D Viruses, p. 1641-1659. In Murray, P. R., Baron, E. J., Jorgensen, J. H., Landry, M. L.,
and Pfaller, M. A. Manual of Clinical Microbiology, 9th ed., vol. 2. ASM Press, American Society for Microbiology, Washington, DC.
James Koziel, M., and Thio, C. L. 2010. Hepatitis B Virus and Hepatitis Delta Virus, p. 2059-2086. In Mandell, D., Bennett, J. E., and Dolin, R. Principles and practice of infectious diseases, 7th ed., vol. 2. Churchill Livingstone, Elsevier, Philadelphia, PA.
HBsAg
| Status | Days | Analytic Time | Maximum Laboratory Time |
Specimen Retention |
| Routine | Mon, Wed, Fri | 7h | 72h | 1 month |
Method Description
The ARCHITECT Anti-HBs assay is a two-step immunoassay, using chemiluminescent microparticle immunoassay (CMIA) technology, for the quantitative determination of anti-HBs in human serum and plasma. In the first step, sample and recombinant HBsAg (rHBsAg) coated paramagnetic microparticles are combined. Anti-HBs present in the sample binds to the rHBsAg coated microparticles. After washing, acridinium-labeled rHBsAg conjugate is added in the second step. Following another wash cycle, Pre-Trigger and Trigger Solutions are added to the reaction mixture. The resulting chemiluminescent reaction is measured as relative light units (RLUs). A direct relationship exists between the amount of anti-HBs in the sample and the RLUs detected by the ARCHITECT i System optics. The concentration of anti-HBs in the specimen is determined using a previously generated ARCHITECT Anti-HBs calibration curve. If the concentration of the specimen is greater than or equal to 10.0 mIU/mL, the specimen is considered
reactive for anti-HBs.
Architect Anti-HBs assay is Health Canada Licensed, 2004.
Performing Laboratory Location
Newfoundland & Labrador Public Health Laboratory
St. John’s