HCV RNA (Hepatitis C virus RNA nucleic acid amplification test)
Useful For
Detection of HCV viremia.
Testing Algorithm
Reporting Name |
Available separately |
Always performed |
| HCV RNA | YES | YES* |
*Only if Anti-HCV positive
Indications for Testing
Detection of hepatitis C virus RNA (viremia status).
Diagnostic indications:
– Indeterminate anti-HCV serological results
– REACTIVE anti-HCV assessment of viremia (autoreflex testing by labortaory)
– Follow-up of infants born to HCV-infected mothers (at 2- 6 months of age)
– Acute HCV infection suspected prior to seroconversion (seroconversion typically occur only after 4 – 6 months)
– Immunosuppressed patient (non-seroconverting)
Response to antiviral therapy indications:
— Week 12 early virologic response assessment (for genotypes other than genotype 2
and genotype 3)
— At end of treatment:
Week 24 (genotype 2 and genotype 3)
Week 48 (genotypes 1, 4, 5, 6)
— Post treatment (detection of relapse) at 6, 12, 24 and 36 months after end of treatment
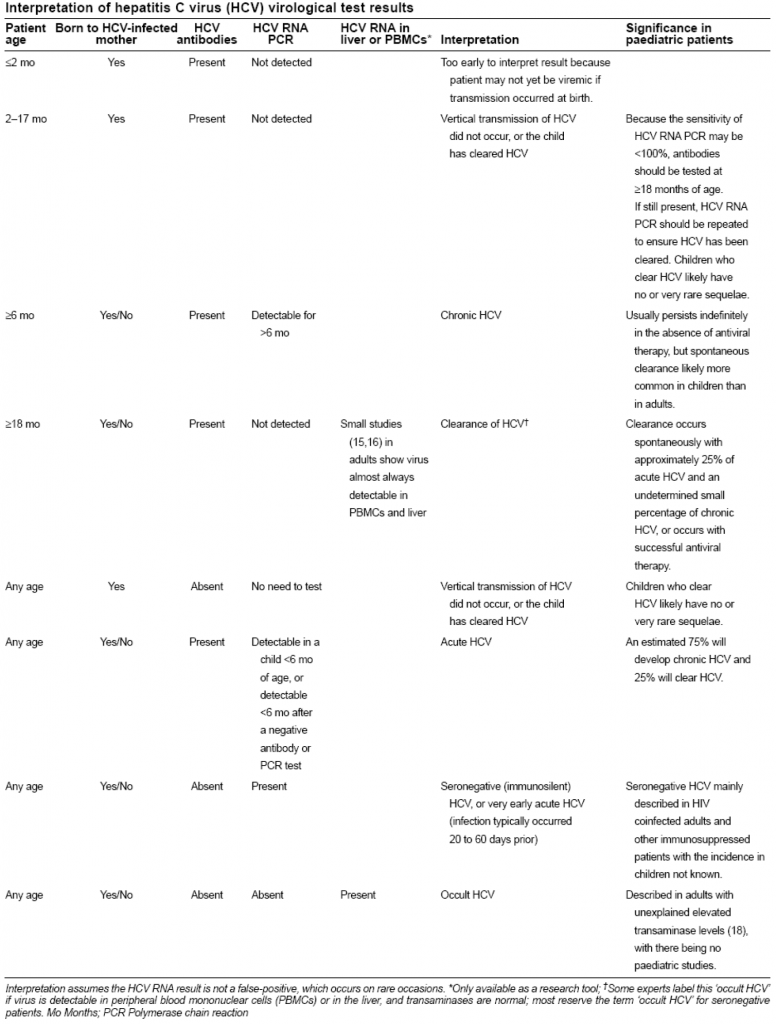
For a child born of an HCV-infected mother
HCV serology is not reliable during infancy because passively transferred maternal antibody may persist for up to 18 months. Therefore serology performed at 12 – 18 months of age is the primary diagnostic test. In special situations of heightened anxiety a single HCV RNA PCR test at a minimum of two months is recommended.
Special Instructions and Forms
Link
Clinical Information
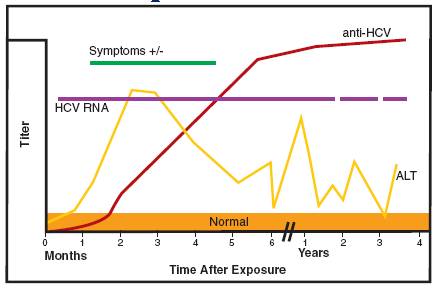
Hepatitis C virus PCR is useful in establishing the diagnosis of acute/active HCV infection in seropositive individuals. HCV RNA PCR may also be used to diagnose acute infection in seronegative individuals because HCV RNA can be detected as early as 1 week after exposure via needlestick or transfusion and at least 4 – 6 weeks prior to seroconversion.
Given the asymptomatic nature of most acute HCV infections, the majority of patients present symptomatically, in the chronic phase. The diagnosis of chronic HCV infection is established with antibody screening tests to document infection and HCV RNA PCR to document virus replication. HCV RNA PCR is particularly advisable for those patients with potential impaired humoral immunity before excluding the diagnosis of chronic HCV infection.
Hepatitis C virus (HCV) infection is diagnosed employing an initial screening for anti-HCV antibodies. The presence of anti-HCV antibodies is indicative of previous or current HCV infection. Determination of active infection is required as >50% of infected, anti-HCV-REACTIVE, patients spontaneously clear the virus. Clearance usually occurs within 14 weeks of exposure, most patients clear the virus within 12 weeks. All anti-HCV REACTIVE specimens are subjected to HCV RNA PCR to determine if circulating virus is present.
HCV antibodies are usually not detected during the first 2 months following infection and are almost always detectable by the late convalescent stage (4-6 months after onset) of infection. These antibodies do not neutralize the virus, and they do not provide immunity against re-infection. Loss of HCV antibodies may occur several years following resolution of infection.
Evolution of HCV infection markers over time.
Most infected people are asymptomatic with onset of disease being insidious. Symptoms range from anorexia, fatigue, fever, myalgia, nausea and vomiting progressing to jaundice. More than 50% of those infected will develop chronic HCV infection. Of those chronically infected about half will develop cirrhosis or cancer of the liver.
Hepatitis C virus (HCV) is an RNA virus of the Flaviviridae family, previously known as NANB hepatitis, which is spread predominantly by parenteral routes. An estimated 170 million people are infected worldwide, and HCV infection is now the leading cause for liver transplantation in the United States because of its propensity to cause chronic liver disease, cirrhosis, and hepatocellular carcinoma.
HCV is recognized as the cause of most cases of post-transfusion hepatitis and is a significant cause of morbidity and mortality worldwide. HCV infection has been reported in virtually every country where it has been carefully evaluated, suggesting that HCV, unlike HIV, has a long-standing global distribution. Occurrence of HCV worldwide is directly related to the prevalence of people who routinely share injection equipment. Approximately 250,000 Canadians are infected with HCV. In Newfoundland and Labrador in 2006 there were 90 cases of HCV reported with a calculated incidence rate of 17.8 per 100,000. The risk factor most commonly reported is injection drug use.
Despite the value of serologic tests to screen for HCV infection, several limitations of serologic testing exist:
1. There may be a long delay (up to 6 months) between exposure to the virus and the development of detectable HCV antibodies.
2. False-reactive screening test results can occur.
3. A reactive screening test result does not distinguish between past (resolved) and chronic HCV infection.
4. Serologic tests cannot provide information on clinical response to anti-HCV therapy.
Pediatric HCV
Unfortunately, HCV serology is not reliable during infancy because passively transferred maternal antibody may persist for up to 18 months. Therefore, serology performed at 12 – 18 months of age is the primary diagnostic test (with the test repeated at 18 months of age, if it is still REACTIVE before that age). Where there is significant parental anxiety about the possibility of HCV infection a single HCV RNA PCR at a minimum of two months of age is recommended, keeping in mind that clearance of the virus can be expected to occur eventually in approximately 25% of viremic infants. If the initial HCV RNA PCR proves positive, then the infant will require testing for HCV RNA and aminotransferase levels every 6 months to determine if chronic infection or spontaneous clearance will ensue. If the initial HCV RNA PCR is negative, serology performed at 12 – 18 months of age to confirm seroreversion.
Reference Values
NOT DETECTED
Interpretation
DETECTED: Hepatitis C virus RNA detected indicative of viremia and infectiousness.
NOT DETECTED: Hepatitis C virus RNA was not detected indicating absence of viremia. A single negative HCV RNA PCR should not be used to exclude viremia. A repeat HCV RNA PCR should be ordered to confirm absence of intermittent viremia.
See Special Instructions for PHL recommended diagnostic approach to hepatitis.
Pediatric interpretation
(Canadian Pediatric Society Position Statement, 2008)
Clinical Reference
Roche Diagnostics. 2001. Cobas Amplicor HCV test v2.0: package insert. Roche Molecular Systems Inc. Branchburg, USA.
Curry, M. P., and Chopra, S. 2010. Acute Viral Hepatitis, p. 1577-1592. In Mandell, D., Bennett, J. E., and Dolin, R. Principles and practice of infectious diseases, 7th ed., vol. 2. Churchill Livingstone, Elsevier, Philadelphia, PA.
Ray, S. C., and D. L. Thomas. 2010. Hepatitis C, p. 2157-2185. In Mandell, D., Bennett, J. E., and Dolin, R. Principles and practice of infectious diseases, 7th ed., vol. 2. Churchill Livingstone, Elsevier, Philadelphia, PA.
Foreman, S.F., and Valsamakis, A. 2011. Hepatitis C virus, p. 1437-1452. In Versalovic J., Carroll C.K., Funke G., Jorgensen J. H., Landry M.L., and Warnock D.W. Manual of Clinical Microbiology, 10th ed., vol. 2. ASM Press, American Society for Microbiology, Washington, DC.
Sherman, M., S. Shafran, K. Burak, et al. 2007. Management of chronic hepatitis C: Consensus guidelines. Can J Gastroenterol 21:25C-34C.
Infectious Disease and Immunization Committee, Canadian Pediatric Society. 2008. Vertical transmission of the hepatitis C virus: Current knowledge and issues. Pediatr Child Health. 03(6):529-534.